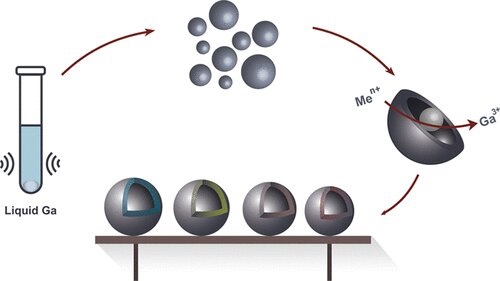
Chemists have succeeded in creating hollow nanoparticles using liquid metals. The new method will not only make these particles easier to produce, but it will also allow the properties of metal nanocapsules to be modified.
Scientists have suggested an alternative method for the production of metal nanocapsules from transition metals based on the galvanic replacement reaction. These hollow nanocapsules are widely used for various purposes: from targeted drug delivery to catalysis induction in petrochemistry.
The conventional methods of producing hollow nanoparticles are complex and expensive: Researchers most often use precious metals such as platinum, silver, or gold. Researchers \ have succeeded in using liquid metal, namely gallium and its alloy with indium, for the same purpose. Their new method will make the production of non-noble metal hollow nanocapsules significantly simpler and cheaper. A drop of metal warmed up to 30 degrees Celsius and subjected to ultrasound produces micro-and nanodroplets. Then, these micro-and nanodroplets are subjected to the galvanic replacement reaction, resulting in hollow metal particles.
They can produce monometallic hollow particles just as well as bi- or even trimetallic ones, They can use additional substances to control the particles’ properties, such as making them less smooth, equipping them with offshoots that increase their total area, making them more or less porous, or changing the thickness of their walls. It’s a versatile method. It will allow any researcher to create a capsule of a specific shape and size, fit for the needs of whichever experiment they’re planning.
Another advantage of liquid metals is that they are relatively inactive. For that reason, similar procedures could potentially be carried out with more than 20 other metals that possess higher reduction potential than gallium and indium in the electrochemical activity series.