There are a handful of ways to produce hydrogen fuel without emitting carbon into Earth’s atmosphere. One involves using electricity to split water into hydrogen and oxygen.
This method, known as electrolysis, requires a catalyst that speeds up chemical reactions that occur within hydrogen fuel cells.
More often than not, this electrocatalyst is platinum, a metal so rare that it’s typically more expensive than gold, which makes the production process more costly than traditional sources of renewable energy and fossil fuels.
Recently, scientists have been studying a lower cost alternative called molybdenum disulfide, which is a two-dimensional compound used in motorcycle engine lubricants and other products. While promising, it’s not nearly as efficient as platinum.
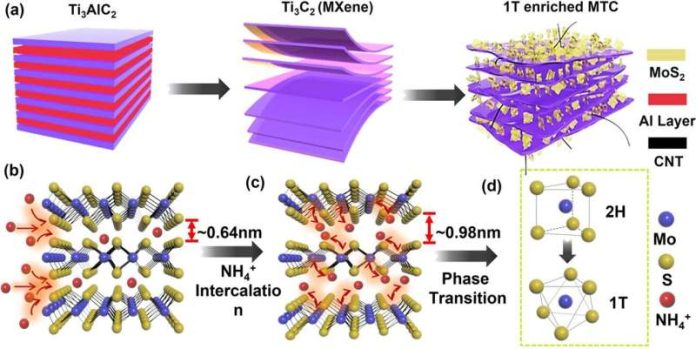
A Study at the University at Buffalo suggests that molybdenum disulfide, when enriched with two additional materials (a class of inorganic compounds known as MXenes and carbon nanotubes), has the potential to supplant platinum as an electrocatalyst, allowing for the more widespread adoption of hydrogen in fuel cell electric vehicles, electricity production and other applications.
Hydrogen has great potential as a clean fuel source. But for that to happen, we must reduce its production cost. This is a step toward that goal.
In the study, researchers describe a one-step chemical reaction, known as solvothermal synthesis, that they employed to add both titanium carbide (the MXene) and carbon nanotubes to molybdenum disulfide.
The resulting ternary structure showed, according to the study, synergistic effects for active site exposure, surface area enlargement and electrical conductivity—all key factors that improve the performance of a catalyst.
“The titanium carbide excels as a conductive backbone, and the carbon nanotubes form a crosslink between the two-dimensional molybdenum disulfide. The combination of all three creates an elegant structure that clearly improves molybdenum disulfide’s performance as an electrocatalyst.
Additionally, the integration of titanium carbide with molybdenum disulfide helps prevent the titanium carbide from oxidizing and it reduces the potential of 2D layer restacking—characteristics that promote catalytic stability.
As a result, the ternary structure demonstrated remarkable catalytic performance improvement compared to other molybdenum disulfide-based electrocatalysts.