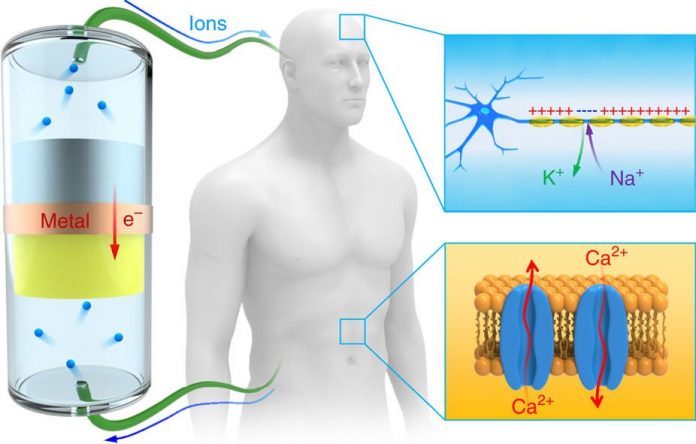
UMD engineers have designed a new type of ion current battery that’s completely biocompatible. The new battery produces the same ion-based energy used by humans and other living organisms, and those ions (in the form of sodium, potassium and other electrolytes) are the electrical signals that do everything from powering the brain to flexing muscles.
To get a better sense of what’s going on here we need to look at how a traditional (electrochemical) battery functions- in this case, chemical reactions from an electrolyte causes a buildup of electrons on the anode (negative), resulting in a difference between the cathode (positive). Think of it as an unstable buildup of electrons, which want to rearrange themselves to get rid of said difference but the only place to go is the cathode. That electrolyte keeps those electrons from doing so until the gap between the anode and cathode is bridged, thereby completing an electrical circuit. The new biocompatible battery, on the other hand, uses electron movement to produce a flow of ions to generate power.
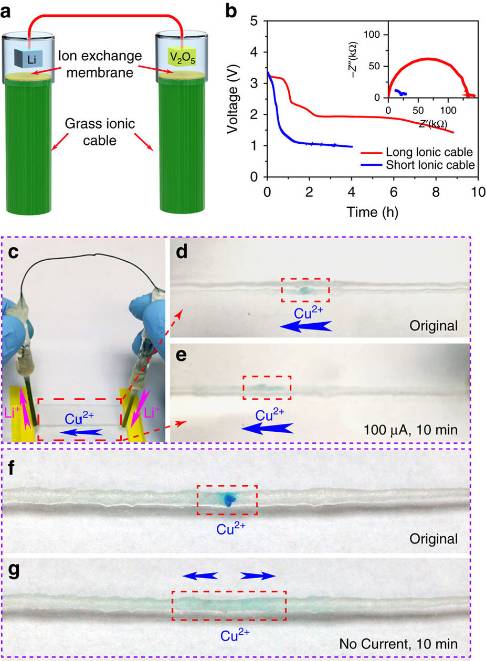
UMD’s professor of materials Liangbing Hu, who headed the battery’s development describes how it functions compared to a traditional electrochemical battery, stating, “In our reverse design, a traditional battery is electronically shorted (that means electrons are flowing through the metal wires). Then ions have to flow through the outside ionic cables. In this case, the ions in the ionic cable – here, grass fibers — can interface with living systems.”
Yes, you read that right, the new battery uses grass as the medium to store energy rather than an electrolyte. More accurately, it uses Kentucky bluegrass coated with a lithium-salt solution, as the channels normally used to move plant nutrients up and down were ideal in holding the ion-producing solution.
The team’s demonstration battery features two glass tubes packed with ion exchange membranes and a blade of solution-soaked grass with both connected together using a thin wire. That wire is where the electrons flow, moving from one end to the other while slowly dissipating energy while a pair of metal tips on the other end of the glass tubes are where the ion current flows.
To prove that ionic flow, the researchers connected the glass tubes at the ends of a lithium-soaked cotton string with a deposition of blue-dyed copper ions placed in the middle. When the current started flowing, that deposition began moving toward the negative charged glass pole, thus proving the ionic current.
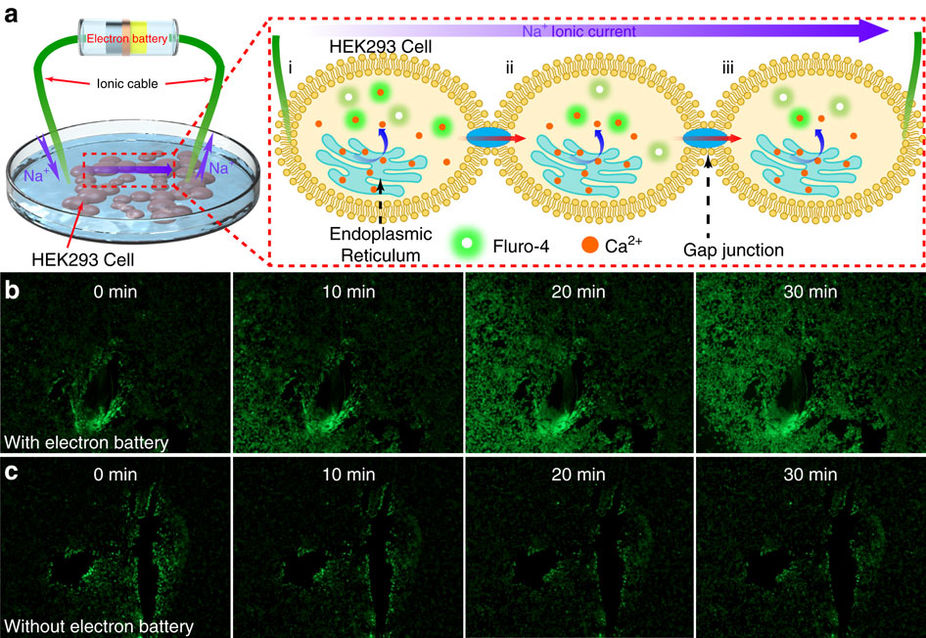
The team has high hopes for their new battery and envision them being used for a number of applications, including the micro-manipulation of neural activities to prevent or treat people with Alzheimer’s disease and depression. They also plan to diversify the types of ion batteries they can produce by using different ionic conductors including cellulose, hydrogels, and polymers.